



| Mast Cell Tumors in Dogs |
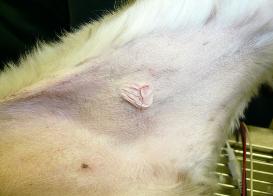
| How common are mast cell tumors in dogs? Skin MCTs are the most common type of mast cell tumors, accounting for 16%-21% of all skin tumors in the dog. The average age at diagnosis is 8 years but puppies as young as 4 months have been reported. There is some evidence that certain breeds may be at higher risk of developing skin mast cell tumors, including boxers, bulldog breeds, bullmastiffs, Boston terriers, Staffordshire, Rhodesian ridgbacks, pugs, Labradors retrievers, golden retrievers, weimaraners, and beagles. Although mast cells are also present in the lung and gastrointestinal tract, the development of tumors in these sites is not as common as for the skin. Skin MCTs are most commonly found on the trunk (50-60%) and the limbs (25%). MCTs that are located underneath the skin appear as soft nodules and can be misdiagnosed as lipoma. Although boxers, bulldogs and pugs are at increased risk of developing skin mast cell tumors, they tend to have less aggressive tumors. Labrador retrievers tend to have more aggressive tumors and golden retrievers tend to have multiple tumors. What are the symptoms of mast cell tumors in dogs? Skin MCTs are usually detected by owners as raised lumps on the skin which can have a wide range of appearance, from a wart-like nodule (mass) to a soft subcutaneous lump to an ulcerated skin mass. MCTs that are located underneath the skin appear as soft nodules and can be misdiagnosed as lipoma. Most tumors are solitary (single mass) but 11-14% of dogs can have multiple tumors. The appearance of these tumors depends on how differentiated the tumor cells are (how much their appearance still resembles mast cells). Tumors that are composed of cells that resemble mast cells under the microscope are usually slow growing and not ulcerated, although the hair may be lost in the area. Tumors that are composed of cells that no longer resemble mast cells are typically rapidly growing and ulcerated. Surrounding tissues may become inflamed and edematous (swollen due to fluid accumulation), with small nodules forming nearby. The clinical symptoms of dogs with MCTs may be complicated due to the release of chemicals by the tumor cells, which can cause gastrointestinal ulceration (open sores). In this case, the dogs may experience vomiting, anorexia, dark feces and abdominal pain. How is the diagnosis made? The World Health Organization has developed a clinical staging system for canine mast cell tumors, reflecting the natural progression of the disease. This system is by no means absolute and serves only as a guide. Mast cell tumors are initially diagnosed by fine-needle aspriation (FNA) cytology. While FNA is useful, it does not provide any information about the tumor's grade (level of aggressiveness), and biopsy is therefore recommended. Additional diagnostic steps will vary depending on the tumor's location, size and initial findings. If the tumor is located at a site easily accessible for surgical removal, and no obvious negative prognostic factors are present, the tumor is usually surgically removed and the sample sent to pathology lab for evaluation. If the tumor develops at a site where complete surgical removal is not possible and/or if additional negative prognostic factors are present, further diagnostic tests are done prior to initiating treatment. The veterinarian will typically examine the regional lymph nodes to check for metastasis (spread of cancer cells), do blood test to check for overall health of the pet, buffy coat smear to check for circulating mast cell tumor cells throughout the body, abdominal ultrasound to check for metastasis to the spleen or liver, chest X-rays to check for metastasis to the lungs and fluid accumulation in the chest, and bone marrow aspiration to check for MCT dissemination (spread). Advanced imaging such as CT (computed tomography) can be a very useful diagnostic tool that can evaluate the extent of the disease in more detail and can aid the oncologist in planning an appropriate treatment strategy. Does cancer cause pain in pets? Pain is common in pets with cancer, with some tumors causing more pain than others. In addition to pain caused by the actual tumors, pets will also experience pain associated with cancer treatments such as surgery, radiation therapy or chemotherapy. Untreated pain decreases the pet's quality of life, and prolongs recovery from the illness, treatment or injury. It is, therefore, essential that veterinary teams that are taking care of pets with cancer should also play a vital role in educating pet owners about recognizing and managing pain in their pets. The best way to manage cancer pain in pets is to prevent it, a term referred to as preemptive pain management. This strategy anticipates pain ahead of time and administers pain medication before the pet actually experiences pain, thus ensuring the pet's maximum comfort. To learn more about which tumors are likely to cause a lot of pain, how to recognize pain in pets with cancer and what cancer pain management options are available for your pet, please visit the Cancer Pain Management section. Is nutritional support important for pets with cancer? Cancer cachexia (a term referring to progressive severe weight loss) is frequently observed in pets with cancer. Pets with cancer lose weight partly because of lack of appetite and partly because of cancer-induced altered metabolism. Some of the causes for decreased appetite are related to the cancer itself (for example, tumors may physically interfere with food chewing, swallowing, and digestion process) and some may be related to the side effects of cancer treatment (for example, some chemotherapy drugs cause nausea and vomiting, and radiation therapy can cause mouth inflammation). Proper nutrition while undergoing cancer treatment is essential to maintain your pet's strength, improve survival times, quality of life and maximize response to therapy. Adequate nutritional support was shown to decrease the duration of hospitalization, reduce post- surgery complications and enhance the healing process. Additionally, pets with cancer need to be fed diets specifically designed to provide maximum benefit and nutritional support for the patient. To learn more, please visit the Cancer Nutrition section. What are the treatment options for dogs with mast cell tumors? Treatment strategies will largely depend on the tumor's grade (level of aggressiveness) and how advanced the disease is. In addition to treating the tumor itself, some cases may also require treatment with anti-histamine medication and/or drugs to relieve gastrointestinal ulceration associated with MCTs. Low or intermediate grade (mildly aggressive) MCT easily accessible for complete surgical removal For easily accessible MCTs, surgical removal is the treatment choice, followed up by histologic evaluation to ensure completeness of surgery. The surgery will typically remove not only the tumor itself but also normal tissue surrounding the tumor to ensure that no cancer cells are left behind. If the histologic evaluation confirms that no cancer cells were present in the healthy tissues, then the dogs should either come for routine check ups on regular basis to monitor possible tumor recurrence/metastasis or the pet owners can agree to chemotherapy. In this setting, the goal of chemotherapy would be to prevent or minimize the risk of metastasis (cancer spread) and/or local tumor recurrence. Because of the lack of adequate studies, veterinary oncologists seem to disagree on the potential benefit of chemotherapy in this particular scenario. If the histologic evaluation confirms the presence of cancer cells in the healthy tissues, then additional treatments are necessary to prevent the tumor from growing back and spreading to distant sites. These will typically include either additional surgery that will remove larger area around the tumor and/or radiation therapy and/or chemotherapy. Recently, the arsenal of anti-cancer therapies for canine mast cell tumors included targeted therapies as summarized in more detail in the next section. Low or intermediate grade (mildy aggressive) MCT not easily accessible for complete surgical removal For tumors that are not easily accessible for complete surgical removal such as distal extremities (eg paws), it is usually recommended that an incisional biopsy is done prior to deciding on a definitive treatment plan. In this case, three main treatment options exist: amputation, radiation therapy and combination of surgery with radiation therapy. Amputation is an aggressive treatment option with the best chance of controlling the tumor but it will also affect the dog's function. Radiation therapy alone has had varying degrees of success. Combining radiation with surgery offers an attractive alternative to limb amputation, in which the surgery removes as much of the mass as possible and the radiation beam kills any remaining cancer cells left behind. Alternative treatments may include surgical removal followed by chemotherapy or chemotherapy alone but the benefit of these treatments is not established. Regardless of the procedure chosen, the dogs should be evaluated on regular basis to monitor for local tumor recurrence and possible metastasis. Recently, the arsenal of anti-cancer therapies for canine mast cell tumors included targeted therapies as summarized in more detail in the next section. High grade (highly aggressive) MCT easily accessible for complete surgical removal For easily accessible MCTs, complete surgical removal is the treatment of choice, followed up by histologic evaluation to ensure completeness of surgery. The surgery will typically remove not only the tumor itself but also surrounding normal tissue to ensure that no cancer cells are left behind. If the histologic evaluation confirms that no cancer cells were present in the healthy tissues, then chemotherapy is used to try to kill any circulating tumor cells that are likely to be present due to the aggressive behavior of high grade tumors. The dogs are then followed up on regular basis for any signs of local recurrence and/or metastasis development. If the histologic evaluation confirmed the presence of cancer cells in the healthy tissues, then either another surgery is attempted that will excise a larger region around the tumor or the area can be subjected to radiation therapy, followed by chemotherapy. High grade (highly aggressive) MCT not easily accessible for complete surgical removal For tumors that are not easily accessible for complete surgical removal such as distal extremities (eg paws), several options are available:
Several chemotherapy drugs have been evaluated for their efficacy. There is accumulating evidence in both human and veterinary oncology that certain combination of different classes of chemotherapy drugs may offer the best chance of controlling the spread of the tumor throughout the body compared to single drugs. The following table summarizes the response to commonly used chemotherapy drugs in the setting of mast cell tumors: Source: Withrow Stephen J, and David M. Vail. Small Animal Clinical Oncology. St Louis: Saunders Elsevier, 2007. Targeted therapies for Grade II/III mast cell cell tumors Advances in scientists' understanding of cancer has led to the development of two targeted therapies for dogs with mast cell tumors: Palladia by Pfizer Animal Health and Masivet (Europe)/Kinavet (U.S.) by AB Science. Palladia (toceranib phosphate, Pfizer Animal Health) Palladia specifically inhibits several molecular targets: proteins called c-KIT, vascular endothelial growth factor receptor-2 (VEGFR- 2), and platelet-derived growth factor receptor (PDGFR-beta), which are known to play a role in cancer. Palladia was evaluated in a multi-center, randomized, placebo-controlled clinical trial in 153 dogs with mast cell tumors that recurred (came back) after surgery. In this study, dogs were randomized to either receive Palladia (given orally at 3.25 mg/kg dose) or placebo (inactive substance serving as a control) every other day for six weeks. During this time, neither the veterinary oncologist nor the owner knew which pill was administered (Palladia or placebo) - a term known as blinded study - to ensure that no bias is introduced and the study is well controlled. The results showed that 32 of 86 dogs (37.2%) who received Palladia responded to treatment, with seven dogs achieving complete response and 25 dogs achieving partial response. In contrast, only five of 63 dogs (7.9%) treated with placebo achieved partial responses. This difference in response between Palladia and placebo was statistically significant. The median time to progression was longer for dogs treated with Palladia compared to those treated with placebo (>6 weeks versus 3 weeks, respectively); and more dogs progressed on placebo versus Palladia during the 6-week blinded phase of the study (66.7% vs. 34.9%, respectively). Dogs with c-Kit mutations were more likely to respond to Palladia compared to dogs with no c-Kit mutation (60% vs. 31.3%, respectively). Dogs who were in the placebo group could receive Palladia after the blinded phase of the study ended, making the total number of dogs treated with Palladia 145. Of these 145 Palladia-treated dogs, 42.8% responded to treatment (21 dogs achieved complete response and 41 dogs achieved partial response). Among the 62 dogs who responded to therapy, median duration of response was 12 weeks and time to tumor progression was 18.1 weeks. Side effects (any severity) that occurred more frequently in dogs receiving Palladia compared to placebo included diarrhea (46% vs 26.6%), bloody stool (12.6% vs. 3.1%), weight loss (14.9% vs. 3.1%), and neutrophil toxicity (46% vs. 6.3%). The full clinical trial results were published in a research journal (London, Clin Cancer Res, 2009), and can be accessed by clicking on the link. Client information sheet about Palladia's safety and efficacy can be found here. Masitinib (AB Science) Masitinib is currently available in Europe under the name Masivet, and in the United Sates under the name Kinavet. Masitinib specifically inhibits several molecular targets: c-Kit, PDGFR alpha and beta, and Lyn. In a clinical trial with 202 dogs of different breeds, dogs with mast cell tumors were randomized to either receive the drug (12.5 mg/kg) or placebo (inactive control). The results of the study showed that masitinib was safe and effective in dogs with Grade II/III skin mast cell tumors. In dogs whose mast cell tumors could not be removed by surgery, masitinib was better than placebo in delaying the time it took for the tumor to progress. Median time to tumor progression for masitinib was 173 days compared to 75 days for dogs treated with placebo. This result was statistically significant (p=0.001). Masitinib also showed a trend toward improving overall survival (617 days for dogs treated with masitinib versus 322 days for dogs treated with placebo; p=0.078), but this result did not reach statistical significance (a statistical significance is usually considered at p value of <0.05). For dogs with tumors harboring a mutation in c-KIT tyrosine kinase receptor, the efficacy of masitinib was even greater compared to placebo in terms of prolonging time to tumor progression (230 days for masitinib versus 42 days for placebo, p=0.006), and median overall survival time (160 days for masitinib versus 62 days for placebo, p=0.025). The most common side effects of masitinib are gastrointestinal reactions (diarrhea and vomiting), which are usually mild to moderate and temporary, although a few may last for up to four weeks. Dogs who receive masitinib should be monitored for side effects by their veterinarian at least once monthly, and if side effects do occur, the veterinarian may either lower the dose or decide to discontinue the treatment. Masitinib cannot be used in dogs with certain liver and kidney conditions, or anemia (low red blood cel count) or neutropenia (low white blood cell count). Additionally, masatinib cannot be used in dogs <6 months old or less than 4kg in weight, or pregnant or nursing female dogs. For more information about masitinib from AB Science, please visit www.masivet.com How do I find a qualified veterinary oncologist? To locate a qualified veterinary oncologist worldwide who can discuss with you appropriate cancer treatment plan for your pet's cancer condition, please visit the "Locate a veterinary oncologist" section. Are there any clinical trials investigating new treatments for mast cell tumors in dogs? There are some clinical trials ongoing for dogs with mast cell tumors in dogs, which are partially funded by the institutions. To learn more about these trials, please visit the Clinical Trials for Mast Cell Tumors in Dogs section. Additionally, there are several clinical trials available for cats and dogs with any tumor type for which your pet may qualify. To learn more these trials (which are partially or fully funded by the institutions), please visit the Dog Clinical Trials (any tumor type) or Cat Clinical Trials (any tumor type) section. To learn more about veterinary clinical trials in general, please visit the Pet Clinical Trials section. What is the prognosis of mast cell tumors in dogs? The prognosis for dogs with MCTs is dependent on a variety of different factors such as the tumor's size, location, grade (level of aggressiveness), age, or symptoms. Tumor grade appears to be the most consistent prognostic factor for dogs with MCTs and the survival times associated with specific tumor grades are summarized the following table. High grade, undifferentiated tumor corresponds to a highly aggressive tumor and most dogs die within 1 year after surgery as a result of local recurrence or metastasis. . Source: Withrow Stephen J, and David M. Vail. Small Animal Clinical Oncology. St Louis: Saunders Elsevier, 2007. Dogs diagnosed with visceral MCTs (tumors in their internal organs) face poorer prognosis compared to those in the skin. These dogs may show symptoms such as anorexia, vomiting, dark feces, wide-spread inflammation, and edema due to the release of substances from the tumor mast cells. One study showed that only 40% of dogs with gastrointestinal MCTs were alive 30 days after diagnosis and less than 10% were alive at 6 months. Therefore, an aggressive treatment is warranted upon the initial diagnosis to improve the pet's long-term prognosis. Additional online resources about mast cell tumors:
Sources:
|

|
|
| PET CANCER CENTER Comprehensive guide to cancer diagnosis and treatment in cats and dogs |
© 2007 Pet Cancer Center. ALL RIGHTS RESERVED.
Last updated 2/21/2017





|
| PET CANCER CENTER CAN ONLY ACHIEVE ITS GOAL IN MAINTAINING THIS COMPREHENSIVE WEBSITE WITH THE ASSISTANCE OF GENEROUS DONATIONS FROM THE PET OWNER AND VETERINARY ONCOLOGY COMMUNITY. WITHOUT THESE DONATIONS, THIS WEBSITE WOULD NOT BE POSSIBLE. PLEASE HELP US REACH OUR GOAL SO THAT THE WEBSITE DOES NOT SHUT DOWN. |
| What are mast cell tumors? Mast cells are present in most tissues, and are especially prominent in the skin, lining of the lungs and digestive tract, mouth and nose. They are an important component of the immune system, with functions in inflammation and allergy reactions. Mast cell tumors (MCTs) are formed by the abnormal proliferation of mast cells, and when the entire body is affected, the disease is referred to as mastocytosis. Mast cells can release several biologically active chemicals such as histamine and heparin, which can be very damaging to the body when released in excess by the tumor cells. The behavior or skin mast cell tumors ranges from being benign (non-cancerous) to highly malignant (cancerous). There is a wide variation in the histological pattern of MCTs and the tumor grade (level of aggressiveness) is a strong predictor of how well the dog will do. The metastatic potential (ability to spread to other organs) is not known for MCTs. It is estimated that well-differentiated tumor cells (those that still resemble mast cells) have a low-to-moderate risk of metastasis but undifferentiated tumor cells (those that no longer resemble mast cells) have a high metastatic potential. Most of these tumors spread first to regional lymph nodes and then mainly to the spleen and liver. |
